The Problem(s) with Candida auris
In 2009, the world was entrenched in another pandemic, albeit much smaller in scale as compared to the current COVID-19 pandemic. It was H1N1 or Swine Flu. But this wasn’t the only pathogen to emerge that year. A new Candida species was isolated from the ear of a patient in Japan — Candida auris (C. auris). Auris means “ear” in Latin, hence its name. Growth of this pathogen over the past decade has been exponential and during the pandemic, while we were singularly focused on SARS-CoV-2, C. auris rates (Table 1) were accelerated with several outbreaks reported. In this article, the key problems with this newcomer will be addressed and solutions will be provided.
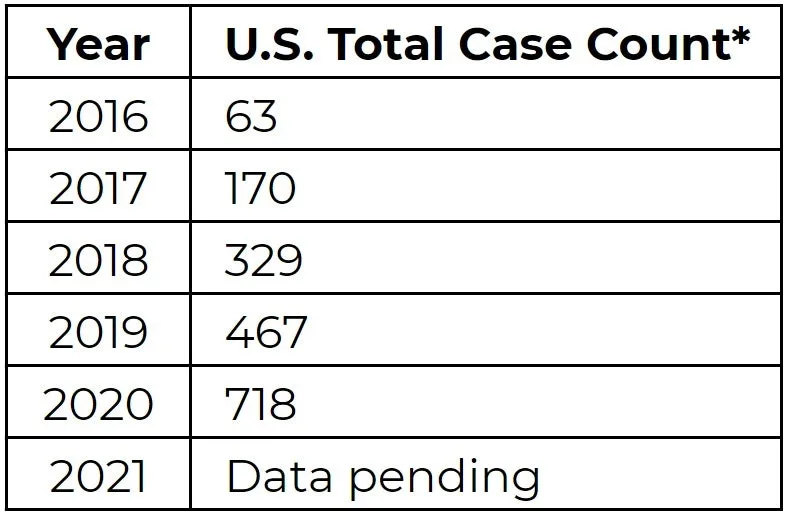
* Clinical cases only (colonization excluded). Table adapted from CDC's Tracking Candida auris.
The Yeast That Behaves Like Bacteria
Candida auris is a unique Candida species in that it behaves more like a bacteria than a yeast and these traits include:
- Multidrug resistance
- Indefinite colonization with no proven decolonization method
- Persistence in the environment where it spreads easily between patients and residents and healthcare facilities
Problem #1: C. auris Is Difficult to Identify
Because C. auris is often misidentified as other Candida species, specialized laboratory technology is required to correctly identify this pathogen. Additionally, many clinical laboratories do not routinely identify yeasts at the species-level when isolated from a non-sterile body site.
Solution: It’s important to know when to suspect and to seek out speciation for C. auris. There are essentially four situations in which this should be done and these are when:
- Yeast isolated from normally sterile body sites such as the bloodstream or cerebrospinal fluid. Speciation guides appropriate initial treatment which can be administered based on the typical, species-specific susceptibility patterns.2
- Candida is isolated from non-sterile body sites. In this scenario, consider species-level identification when:2
- its clinically indicated for the patient’s care
- in the previous year, a patient has had an overnight stay in a healthcare facility outside of the United States
- A Candida species is isolated from surveillance cultures collected when actively looking for additional cases when a case has been detected in a facility or a unit
- A fungal isolate is identified that is known to represent potential misidentification of C. auris, such as C. haemulonii.2
- You are seeing an increase in infections due to Candida species in a given care unit or there has been an increase of Candida isolated from urine specimens.2
This algorithm in Figure 2 may help in determining when to speciate:2,4
Figure 2.
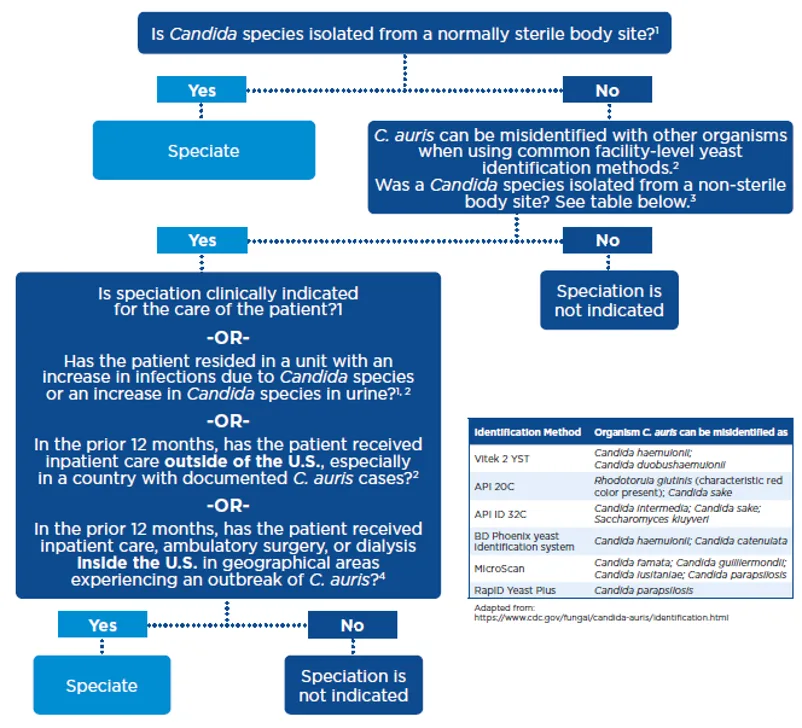
If your facility lab does not have the technology to identify C. auris, the CDC has a really helpful table which summarizes misidentifications by the type of lab test used. The recommendation is that if any of these species are identified or if species level cannot be determined, further characterization using appropriate testing methods should be sought through your local public health dept.2
Problem #2: C. auris Persists in the Environment
Surface transmission is the primary route in which C. auris is spread and once it gets a foothold in a facility, it can be very difficult to eradicate. Colonized patients shed the pathogen into the environment where this pathogen can survive on environmental surfaces for several weeks. Additionally, portable medical equipment has been implicated as a reservoir in several outbreaks.
For these reasons, a robust infection prevention and control plan including cleaning and disinfection is critically important. The CDC recommends the use of EPA-registered disinfectants that have kill claims for either C. auris or C. difficile.2 Use of diluted bleach is another alternative.2 In fact, enhanced cleaning with bleach 3 times daily is how one facility stopped a C. auris outbreak.5 It’s important to note that quaternary ammonium compounds (“quats”) are not as effective against this C. auris.2 Disposable ready-to-use disinfecting wipes should be readily accessible for staff at point of use. And because 50% of surfaces are missed during manual cleaning, use of adjunct disinfection technologies such as electrostatic disinfection systems can help to ensure that nothing gets missed and no C. auris is left behind.
Problem #3_: C. auris_ Spreads Rapidly In and Between Healthcare Facilities
Outbreaks with C. auris have proven challenging to control.This pathogen can spread in healthcare settings through contact with contaminated environmental surfaces, medical equipment, and fomites or from person-to-person such as from unclean hands. Recent investigations have demonstrated that one-third to half of all patients on a given unit can become colonized with C. auris within a matter of weeks of an index case entering the facility.6 Other studies have found that C. auris may be found not only in patient’s rooms but also in hallways, on countertops, and medical equipment - particularly portable equipment. Shared multi-use patient care equipment such as temperature probes and pulse oximeters may act as reservoirs for this drug-resistant fungus_._7 In addition to robust cleaning and disinfection, the CDC recommends contact isolation precautions in addition to standard precautions to contain C. auris.2
Summary
With our attention on a single pathogen during the pandemic — SARS-CoV-2 — a few emerging pathogens like C. auris have been able to get a foot hold in this country. C. auris is a problematic pathogen which has proven to be a very challenging and difficult to contain pathogen. The evidence-based recommendations provided in this article should be considered in the event that you are faced with C. auris.
References
^1. Centers for Disease Control and Prevention. 2019 Antimicrobial Resistance Threats Report. [Internet]. [Cited 2021 Dec 14]. Available from https://www.cdc.gov/drugresistance/biggest-threats.html
2. Centers for Disease Control and Prevention. Candida auris. [Internet]. [Cited 2021 Dec 14]. Available from https://www.cdc.gov/fungal/candida-auris/index.html
3. Hayden M, et al. Characterization of Skin Microbiota, and Relation of Chlorhexidine Gluconate (CHG) Skin Concentration to C. auris Detection Among Patients at a High-Prevalence Ventilator-Capable Skilled Nursing Facility (vSNF) with Established CHG Bathing. Open Forum Infect Dis. 2019; 6(Supple 2): S25-S26.
4. Minnesota Department of Health. Admission Screening Recommendations. [Internet]. [Cited 2021 Dec 14. Available from https://www.health.state.mn.us/diseases/candidiasis/auris/index.html
5. Schelenz S, Hagen F, Rhodes J, Abdolrasouli A, Chowdhary A, et al. First Hospital Outbreak of the Globally Emerging Candida auris in a European Hospital. Antimicrob Resist Infect Control. 2016; 5:35.
6. Council of State and Territorial Epidemiologists. Standardized Case Definition for Candida auris clinical and colonization/screening cases and National Notification of C. auris, clinical. (nd). [Internet]. [Cited 2021 Dec 17]. Available from https://cdn.ymaws.com/www.cste.org/resource/resmgr/2018_position_statements/18-ID-05.pdf.
7. Sikora A, Zahra F. Candida Auris: Continuing Education Activity. 2021. StatPearls [Internet]. [Cited 2021 Dec 17]. Available from https://www.ncbi.nlm.nih.gov/books/NBK563297/^
















